FSVP Enforcement: The #1 Reason for 483s
FSVP Citations
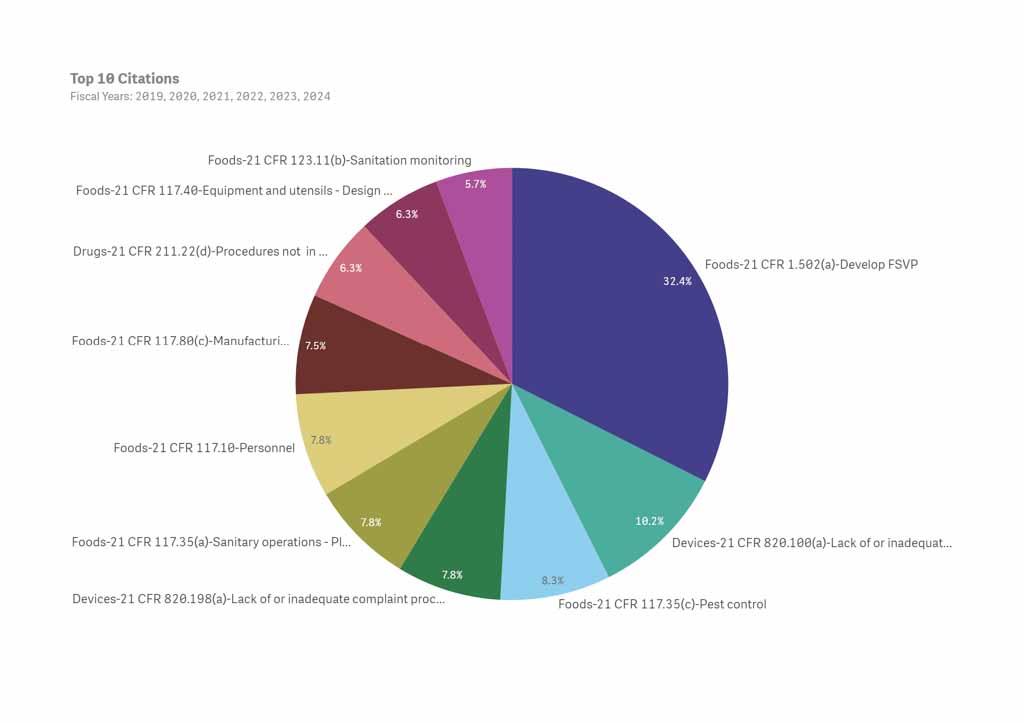
The Foreign Supplier Verification Program (FSVP) rule is enforced by the U.S. Food and Drug Administration (FDA) far more than any other regulation within its authority. The FSVP regulation went into effect in May 2017 and FDA ramped up enforcement in 2019. From Fiscal Year 2019 to FY2024, 32.4 percent of the top ten citations issued by FDA, including those for food, drugs, cosmetics and medical devices, were for FSVP violations. This data may be found on the FDA Data Dashboard.
How often should I expect to be inspected for FSVP compliance?
In our experience importers will be inspected within three to four years of their first importation and about every three years thereafter. FDA conducted more than 700 FSVP inspections in FY2022 and FY2023.
What are the consequences for failing to comply with the FSVP regulation?
Violations of the FSVP rule are punishable with civil and criminal penalties. Importers who fail to comply may receive citations, warning letters, be placed on import alert and can lose the privilege to import.
Can our CEO be held personally liable for FSVP violations?
Yes. Consistent with the U.S. Supreme Court ruling United States v. Park, 421 U.S. 658 (1975), corporate officers can be held criminally liable for violations of the Food, Drug and Cosmetic Act, including FSVP, even if they did not have actual knowledge of the violation.