In This Week’s Edition:
- FSVP Most Heavily Enforced Regulation by FDA for 2021
- FDA Releases New Total Diet Study Report
- FDA Reopens Comment Period on Use of Certain Phthalates in Food Packaging
- Compliance Dates Proposed for Pre-Harvest Water Standards for Produce Safety Rule
- FDA Shares Results on PFAS Testing in Seafood; Identifies Health Concern in Canned Claims Prompting Recall
- AMS Soliciting Comments on Addition of Certain Sugarcane and Squash to List of Bioengineered Foods
- FDA CORE Outbreak Investigation Status Update
- FSIS Updates Lists of Foreign Establishments Eligible to Export to U.S.
- FDA Import Alerts
- FDA Warning Letters
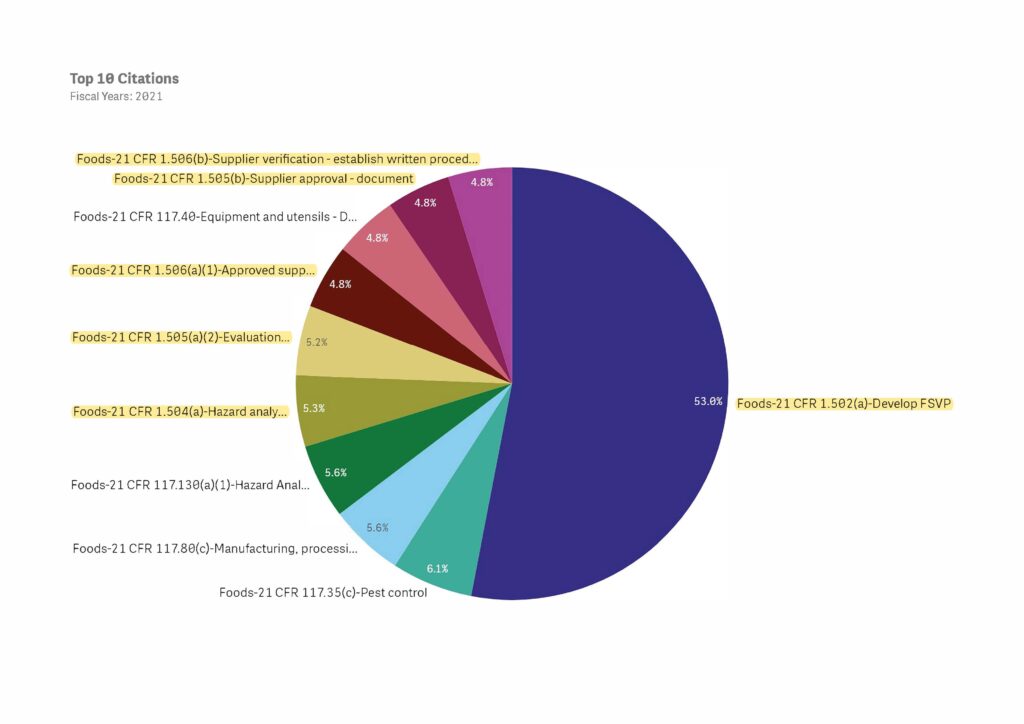
FSVP Most Heavily Enforced Regulation by FDA for 2021
FDA has released their inspection citation data for FY 2021. Nearly 80% of all of FDA’s citations (including foods, drugs, biologics and medical devices) were related to FSVP violations.
U.S. Food Imports provides FSVP compliance services to the food industry, including chain supermarkets, food manufacturers, importer, wholesalers and e-commerce businesses. We create and implement your company’s FSVP program and handle the FSVP inspections when they occur. Please contact us at elieberman@usfoodimports.com or 202.765.1800 for additional information.
FDA Releases New Total Diet Study Report
FDA released the agency’s Total Diet Study (TDS) Report: Fiscal Years 2018-2020 Elements Data and associated data tables. The TDS helps the FDA prioritize food safety and nutrition efforts. This report is the first in a series on FY2018-FY2020 TDS data and summarizes the agency’s most recent data on nutrients and toxic elements from FDA’s on-going survey of the U.S. food supply.
FDA Reopens Comment Period on the Use of Certain Phthalates in Food Packaging
FDA will reopen the comment period for the request for information seeking available use and safety information on the remaining phthalates authorized for use as plasticizers in food contact applications. The request for information appeared in the Federal Register on May 19, 2022, with comments due by July 19, 2022. The new deadline for comments will be published in an upcoming Federal Register notice. The FDA is reopening the comment period in response to a request to provide stakeholders with more time to fully consider the request for information and submit comments.
Compliance Dates Proposed for Pre-Harvest Water Standards for Produce Safety Rule
FDA is proposing dates for compliance with the pre-harvest agricultural water provisions for covered produce other than sprouts in the Produce Safety Rule:
- 2 years and 9 months after the effective date of a final rule for very small businesses;
- 1 year and 9 months after the effective date of a final rule for small businesses;
- and 9 months after the effective date of a final rule for all other businesses.
FDA has proposed an effective date of 60 days after the publication of a final rule.
FDA also clarifies that they intend to continue enforcement discretion for the harvest and post-harvest agricultural water requirements of the Produce Safety regulation until the following dates:
- January 26, 2025, for very small businesses;
- January 26, 2024, for small businesses; and
- January 26, 2023, for all other businesses.
FDA Shares Results on PFAS Testing in Seafood; Identifies Health Concern in Canned Clams Prompting Recall
FDA made available testing results for per- and polyfluoroalkyl substances in seafood samples collected at retail. The FDA conducted this limited survey as a preliminary step to determine if a more targeted or larger seafood survey should be conducted. After seeing the results, a second firm has issued a voluntary recall.
AMS Soliciting Comments on Addition of Certain Sugarcane and Squash Varieties to List of Bioengineered Foods
USDA’s AMS is soliciting comments and feedback on adding certain sugarcane and squash varieties to the List of Bioengineered Foods as it pertains to the National Bioengineered Food Disclosure Standard.
FDA CORE Outbreak Investigation Status Update
This week’s updates are:
- For the Salmonella Braenderup outbreak (ref# 1075), the case count has increased from 63 to 70.
FSIS Updates Lists of Foreign Establishments Eligible to Export to U.S.
FDA Import Alerts
The latest FDA import alerts include dried fruits due to lead, foods due to heavy metal contamination, food containing undeclared/illegal colors, and more.
FDA Warning Letters
| Rockwall Vaporstop LLC |
| Family Smoking Prevention and Tobacco Control Act/Adulterated/Misbranded |
| Young Living Essential Oils Corporate |
| New Drug/Misbranded |
| Genlabs Corporation |
| CGMP/Finished Pharmaceuticals/Adulterated |
| New Sun Inc. |
| Unapproved New Drugs/Misbranded/Cannabidiol (CBD) Products |
| Bioiberica, SAU |
| CGMP/Active Pharmaceutical Ingredient (API)/Adulterated |
| Greenfield Produce Imports |
| Foreign Supplier Verification Program (FSVP) |
| Brad Grate Dairy Farm |
| New Animal Drug/Adulterated |
| Dara Food LLC |
| Foreign Supplier Verification Program (FSVP) |
| Flores Produce, Inc. |
| Foreign Supplier Verification Program (FSVP) |
| North30 LLC |
| Foreign Supplier Verification Program (FSVP) |
| Swan Brothers Dairy Inc |
| CGMP/Food/Prepared, Packed or Held Under Insanitary Conditions/Adulterated/L. monocytogenes |
| H2 Beverages, Inc. |
| Unapproved and Misbranded Products Related to Coronavirus Disease 2019 (COVID-19) |
| Jap Inc. dba Intercontinental Foods |
| Foreign Supplier Verification Program (FSVP) |
| Regino Produce LLC |
| Foreign Supplier Verification Program (FSVP) |
| Free Range Vapor LLC |
| Family Smoking Prevention and Tobacco Control Act/Adulterated/Misbranded |
| USA Vapor Trails |
| Family Smoking Prevention and Tobacco Control Act/Adulterated/Misbranded |
| Imprimis Rx LLC |
| False & Misleading Claims/Misbranded |
| Living Foods LLC |
| Unapproved New Drugs/Misbranded |







